Scale-Up
Streamlined transitions from development to production.
We streamline the scale-up of your processes from R&D to production, ensuring minimal disruption and full alignment with regulatory and operational requirements. Our strategic planning and hands-on oversight help you scale confidently and efficiently.
Streamlined transitions from development to production.
Well-structured, compliant documents ready for audits.
Early identification and mitigation of potential issues.

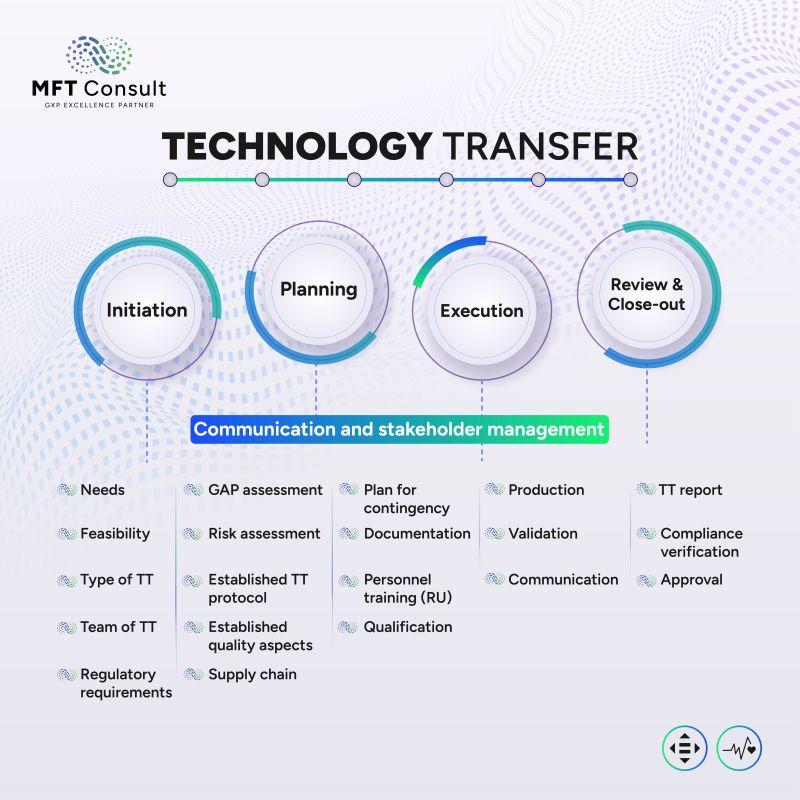
Our service includes critical validation processes and expert coordination to ensure technology transfer success. We focus on aligning manufacturing and analytical standards with regulatory expectations to guarantee compliance and performance.
We don’t just manage your transfer—we prepare your team, systems, and structure for long-term success through informed decision-making and clear project ownership.
By aligning all stages with GMP standards, coordinating teams early, and validating methods and processes before handover.
Common issues include misaligned timelines, unclear responsibilities, or inconsistent documentation. We address these through structured planning and clear governance.
Yes. We offer continued assistance in optimization, troubleshooting, and validation updates to keep operations running efficiently post-transfer.