%
Client Retention Rate

MFT Consult is an international consultancy specializing in regulatory compliance, facility design, and QMS for pharma and biopharma startups and expansions. With global experience and deep regulatory insight, we help clients navigate complex requirements confidently.
Client Retention Rate
Inspection Readiness Success



MFT Consult supports pharma and biopharma startups with tailored GxP and compliance solutions. We drive operational excellence and regulatory readiness across emerging markets. With global expertise and local focus, we help you grow with confidence.
To be the go-to partner for pharma and biopharma companies, driving compliance and innovation.
To support startups and expansions with expert guidance in compliance and quality systems.
Flexible structure ensures quick adaptation and efficient solutions.
Specialized teams provide tailored solutions for client needs.
Culture fosters collaboration, integrity, innovation, and excellence.
We provide specialized project management for seamless technology transfer from development to production, ensuring compliance, quality, and smooth integration of new processes.
Our international experience enables us to provide comprehensive regulatory support. We help your startup comply with WHO, FDA, and EMA standards, while aligning your operations with local regulations and localization goals across the Middle East. From supplier selection to full market entry, we ensure a seamless and compliant pathway.
We assist startups in designing and optimizing GXP-compliant facilities, from initial design to commissioning and validation, ensuring scalability for international markets and supporting local manufacturing initiatives.
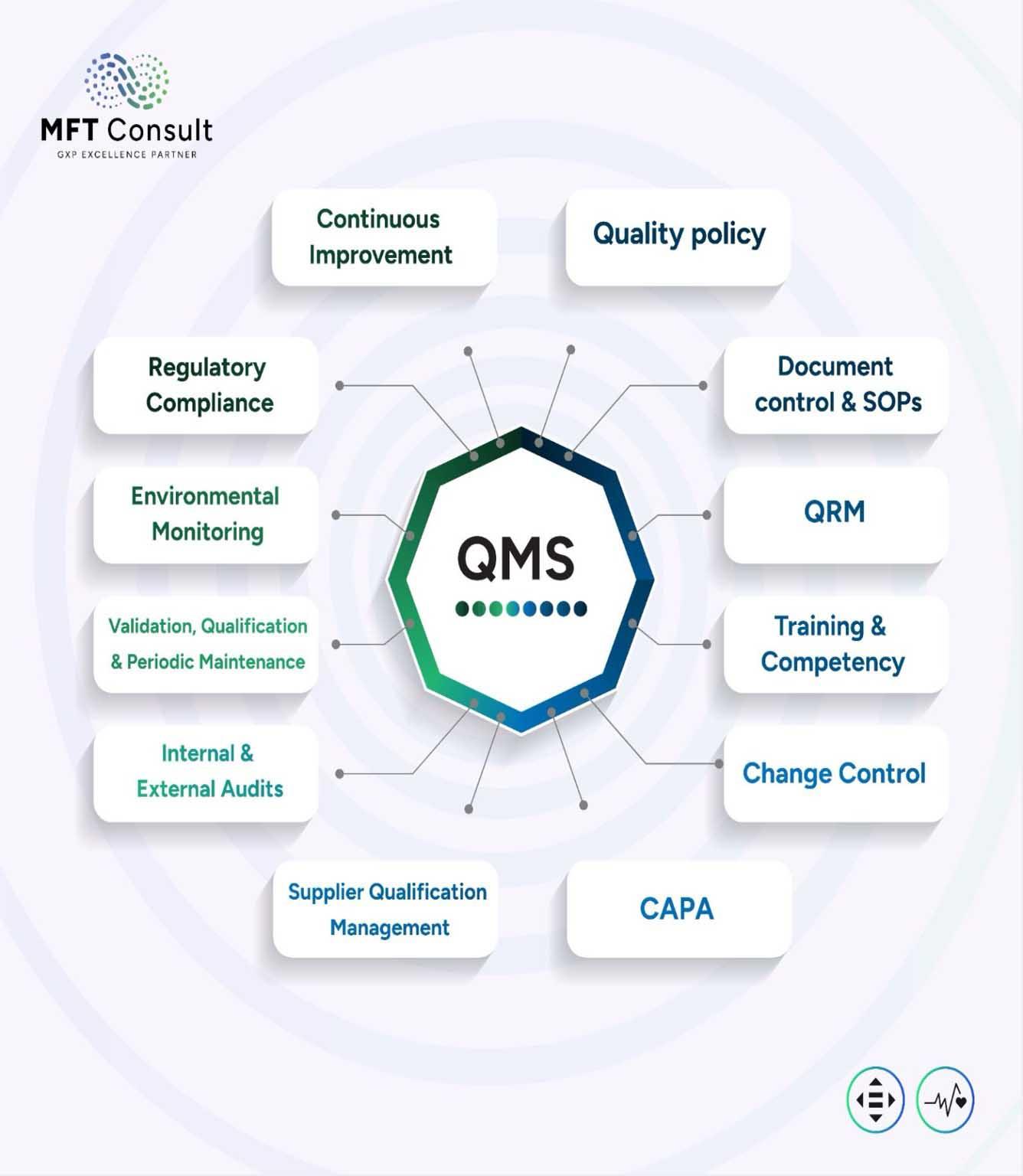
We design and enhance tailored Quality Management Systems (OMS) that ensure global
compliance while supporting the localization of pharmaceutical manufacturing in Africa and the Middle East.
At MFT Consult, we offer specialized services that strengthen your operations beyond core consulting. From documentation setup and risk mitigation to business development and training, our additional services are tailored to support compliance, safety, and sustainable growth at every stage of your journey.
At MFT Consult, we combine technical expertise and industry insight to deliver specialized services that support pharmaceutical innovation, regulatory excellence, and operational success across Africa and the Middle East.
We specialize in navigating international and local regulatory frameworks, ensuring pharmaceutical startups meet WHO, FDA, and EMA standards while adapting to the localization needs of African and Middle Eastern markets.
Our team provides end-to-end support in designing, commissioning, and validating pharmaceutical, biopharma, and clinical facilities to meet Good Manufacturing Practice (GMP) requirements—ensuring operational readiness and long-term scalability.
We develop customized QMS frameworks that align with your operations, incorporating SOPs, CAPA processes, and audit tools to maintain product quality, safety, and regulatory compliance across evolving manufacturing environments.